|
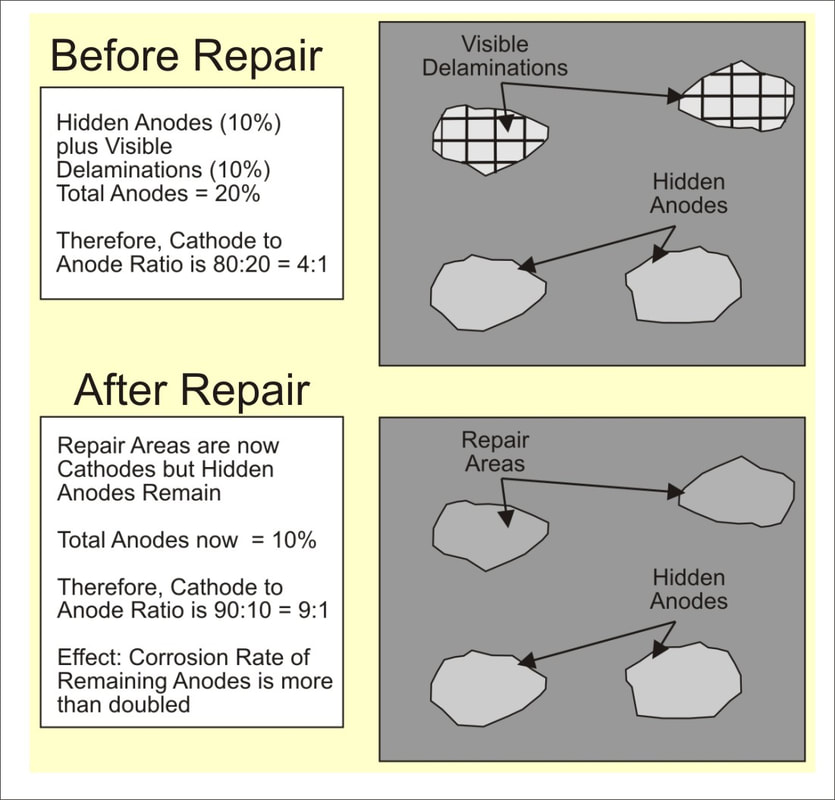
I believe the logical answer to the posed question is that the selected restoration strategy did not achieve electrochemical compatibility of the steel reinforcement network within the repaired deck. This was likely because the concrete repair material accelerated the rate of corrosion of the adjacent rebar, which was corroding, but had not advanced to the stage where it had caused delamination of the concrete.  The full technical reason for this phenomenon - often referred to as the ring or halo effect - is too involved to explain briefly within this blog. However, I’ve always found the Figure I have included below as very helpful in understanding what can happen. Often the approach to restoration is to simply remove visibly deteriorated concrete while ignoring the fact that corrosion will continue within the surrounding concrete. However, the repaired areas will change from anodic to cathodic sites, thereby increasing the remaining cathode to anode ratio - this will increase the rate of corrosion of the remaining anodic sites. (as illustrated by the Figure) For this reason, I believe that the use of corrosion evaluation testing methods in conjunction with a delamination survey can be essential. WHAT IS THE SOLUTION? Experiences both in the laboratory and on restoration projects have convinced me that a very effective and economical way to achieve electrochemical compatibility is to apply a zinc rich epoxy primer to the exposed reinforcing steel within the repair area; the zinc acts as a sacrificial anode, thereby mitigating corrosion activity within the steel.  This and other restoration issue are included in INVESTIGATING CONCRETE PROBLEMS Learning from Those Who Learned the Hard Way!
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorBased in Guelph, Ontario, Paul Jeffs founded PJ Materials Consultants in 1989 to provide specialist consulting services for North American construction-related industries. Prior to this, he lived and worked in several countries, including the U.K., Bahrain, Iran and Japan, and provided technical advice to many construction projects within the regions surrounding those countries. Paul specializes in providing materials-related technology advice for the construction, protection and restoration of concrete and masonry - and especially for the conservation of heritage buildings and structures. Archives
March 2023
Published Books
|
|
PJ Materials Consultants was formed by Paul Jeffs in 1989 to provide a wide range of specialist consulting services for concrete and masonry structures. The main business activity is divided between the provision of materials technology expertise and professional development technical training.
|